
Abiraterone Approved for Earlier Use in Men with Metastatic Prostate Cancer
February 15, 2018, by NCI Staff
Even more men with advanced prostate cancer may benefit from the drug abiraterone (Zytiga®), after the Food and Drug Administration (FDA) expanded the drug’s approval on February 7.
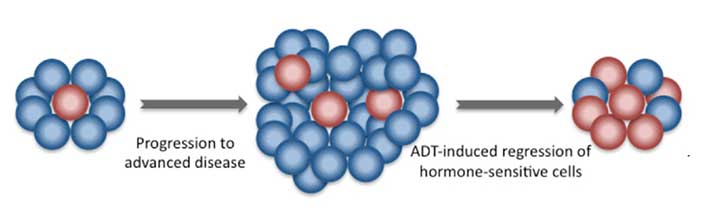
The agency approved abiraterone, in combination with the steroid prednisone, for men with metastatic prostate cancer that is responsive to hormone-blocking treatments (also known as castration-sensitive) and is at high risk of progressing. Abiraterone is already approved by FDA as a treatment for men with metastatic prostate cancer that has developed resistance to hormone-blocking treatments (also known as castration-resistant disease).
The new approval is based on the results of a nearly 1,200-patient clinical trial called LATITUDE that was sponsored by Janssen Pharmaceuticals, which manufactures abiraterone.
In the trial, patients were randomly assigned to receive either abiraterone and prednisone daily, along with standard hormone-blocking treatments called androgen-deprivation therapy (ADT), or ADT alone. Men treated with abiraterone and prednisone lived longer overall and without having to initiate chemotherapy to help control metastasis-related pain.
In two other recent clinical trials, CHAARTED and STAMPEDE, the chemotherapy drug docetaxel, in combination with ADT, also improved overall survival in men with castration-sensitive metastatic prostate cancer.
Updated Data Published on Docetaxel’s Survival Benefit in Advanced Prostate Cancer
In some men with castration-sensitive metastatic prostate cancer, adding docetaxel to ADT improves how long they live overall. The finding comes from longer-term follow-up of participants in the NCI-funded CHAARTED clinical trial.
Interim results from the trial, released in 2013, had already shown that adding docetaxel to ADT increased overall survival. Findings from the longer-term follow-up of men in the trial, however, indicate that the survival improvements are limited to men with high-volume disease—that is, cancer that has spread to other organs or to bone in four or more places.
No additional side effects were seen with the extended follow-up, the trial’s lead investigator Christopher Sweeney, M.B.B.S., of Dana-Farber Cancer Institute, and his colleagues reported January 31 in the Journal of Clinical Oncology.
The findings do not definitively mean that some men with low-volume disease “do not benefit” from adding docetaxel to ADT, the researchers wrote. But they do reinforce that “more precise biomarkers” are needed to identify men who are likely to benefit from the drug.
Based on the findings from the CHAARTED, STAMPEDE, and LATITUDE trials, “a combination approach” of docetaxel or abiraterone with ADT “is now considered a standard of care” for men with castration-sensitive metastatic disease, explained Che-Kai Tsao, M.D., and William Oh, M.D., of the Mount Sinai Hospital in New York, in an editorial published February 12 in the Journal of Clinical Oncology.
But because the therapies haven’t been compared against each other in a clinical trial, Drs. Tsao and Oh continued, “it remains unclear who should receive docetaxel, abiraterone, both, or neither.”
As a result, they concluded, “clinicians must rely on noncomparative interpretation and their personal experience to guide treatment decisions.”
Further details on the LATITUDE trial results and how the expanded approval of abiraterone may affect patient care are available in this June 2017 Cancer Currents post.






















.png)











No hay comentarios:
Publicar un comentario